The catalytic converter is a critical emissions control device in a vehicle’s exhaust system. It utilizes precious metal catalysts such as platinum, rhodium, and palladium to convert harmful gases—carbon monoxide (CO), hydrocarbons (HC), and nitrogen oxides (NOx)—into harmless carbon dioxide, water, and nitrogen. Its core working principles include oxidation reactions (converting CO and HC into CO₂ and H₂O) and reduction reactions (breaking down NOx into N₂ and O₂). These processes require efficient operation within high-temperature environments of 400–800°C.
Key Features and Maintenance Guidelines
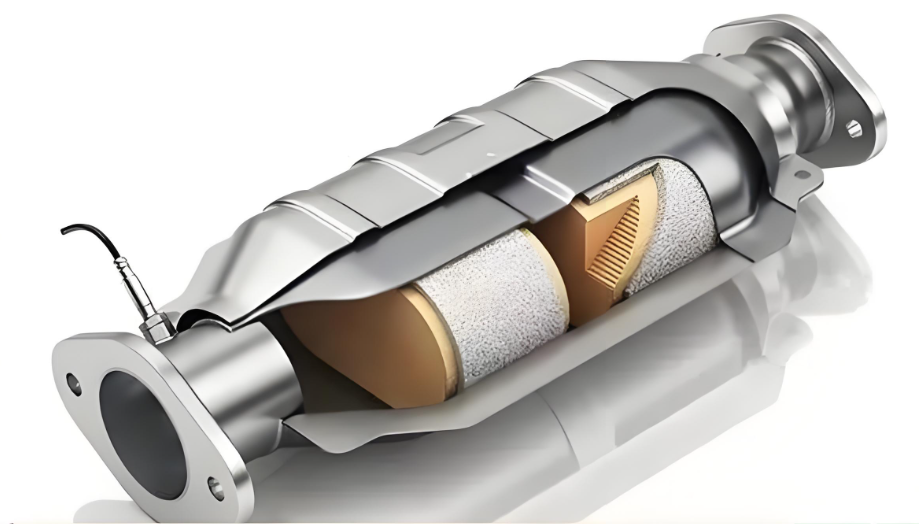
Structural Design
The substrate is typically made of porous ceramic or metal materials, coated with a layer of precious metals to maximize the reactive surface area. Its structure often resembles a honeycomb or a military canteen.
Common Causes of Failure
Clogging: Accumulated carbon deposits or silicon residues (e.g., from low-quality gasoline) can block the micro-channels, increasing exhaust backpressure and reducing engine performance.
Chemical Poisoning: Substances like sulfur and phosphorus can coat the catalyst surface, diminishing its activity.
Thermal Damage: Prolonged engine misfires or poor combustion can lead to overheating, potentially causing the substrate to melt.
Maintenance Recommendations
Regular Inspection: Use an exhaust gas analyzer to monitor HC and NOx concentrations, or physically inspect the converter for clogging.
Cleaning Methods: Clean with specialized chemical agents (avoid fuel tank additives) and rinse with water. Do not use physical cleaning methods to prevent damage.
Preventive Measures: Maintain optimal engine performance and avoid using low-quality fuel.
